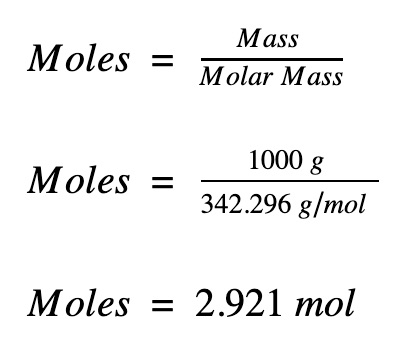How to Accurately Calculate Moles
Understanding the concept of **moles** is fundamental to mastering chemistry. Moles form the backbone of quantitative analysis in chemical reactions, enabling chemists and students alike to convert measurements from the macroscopic world of grams and liters to the microscopic world of individual molecules and atoms. In this guide, we’ll explore the various aspects of how to calculate moles, providing practical examples and actionable insights for enthusiasts in 2025.
Understanding Moles: The Basics
The **mole definition** is fundamental in chemistry. It is a unit used to measure the amount of a substance. One mole of any substance contains exactly \(6.022 \times 10^{23}\) entities (this number is known as **Avogadro’s number**). The **mole formula** encapsulates this relationship: it links the mass of a substance to the number of moles present by utilizing its **molar mass**, which can be found on the periodic table. For example, if the molar mass of carbon is 12 g/mol, it means that 12 grams of carbon corresponds to one mole of carbon atoms.
Mole Calculation: Steps and Examples
To **calculate moles**, you can follow a straightforward formula: n = m / M, where n represents moles, m is the mass of the substance (in grams), and M is the molar mass (in g/mol). For instance, to find the number of moles in 24 grams of NaCl (molar mass of approximately 58.44 g/mol), you calculate: n = 24 g / 58.44 g/mol ≈ 0.41 moles.
Converting Grams to Moles
The process of **converting grams to moles** is crucial in various applications within chemistry. Let’s use sulfuric acid (H₂SO₄) as an example, which has a molar mass of approximately 98.08 g/mol. If you have 196.16 grams of sulfuric acid, you can easily determine the moles by dividing the mass by the molar mass. Hence, n = 196.16 g / 98.08 g/mol = 2 moles. This method is universally applicable across different substances.
Utilizing Moles in Chemical Reactions
Moles are indispensable in the realm of **stoichiometry and moles**. They allow chemists to predict the outcomes of chemical reactions by helping them understand the relationships between different reactants and products. For instance, in the reaction between hydrogen and oxygen to create water (2H₂ + O₂ → 2H₂O), knowing the molarity of each reactant can help calculate how many moles of each substance are needed to produce a certain amount of water.
Moles and Gas Laws
Examining the intersection of **moles in gas laws** can be immensely beneficial for science enthusiasts. The **ideal gas law**, expressed as PV=nRT, explicitly shows how moles relate to pressure, volume, and temperature. Here, **n** represents the number of moles. For example, if you have a gas at a pressure of 2 atm, occupying 10 liters at a temperature of 300 K, you can rearrange the ideal gas law to calculate the moles as follows: n = PV / RT. Substituting the constants gives you a tangible sense of how moles work in gaseous environments.
Molecular Volume and Moles
Understanding the relationship between **moles and molecular volume** is also pivotal. At standard temperature and pressure (STP), one mole of any ideal gas occupies roughly 22.4 liters. This principle allows you to convert from liters to moles using the relationship: n = volume (L) / 22.4 L/mol. This makes gas stoichiometry much more manageable, allowing calculations to be performed easily during laboratory experiments.
Determining Molarity and Concentration
The term **molarity** refers to the concentration of a solute within a solution, defined as moles of solute per liter of solution. To calculate molarity, you could use the formula: M = moles of solute / liters of solution. If you dissolve 0.5 moles of sodium chloride in 2 liters of water, your molarity would be 0.25 M, denoting how concentrated your solution is. Molarity is a crucial aspect of **moles in solution chemistry**.
Practical Applications of Moles
The use of moles extends into various fields, from **chemical biology** to **food science**. In biochemistry, understanding the concentration of substances in cells can be pivotal. For example, calculating the concentration of glucose in a solution can aid in understanding metabolic rates. Furthermore, moles play a critical role in dietary guidelines by allowing nutritionists to convert grams of nutrients into their respective mole equivalents, thereby enabling more precise dietary planning.
Advanced Mole Concepts
Diving deeper into **moles in physical chemistry** reveals several advanced concepts, including percent composition and molecular versus empirical formula calculations. The percent composition involves determining what percentage of a compound’s mass is due to each individual element. By conducting calculations involving the molar masses of each component, you can unlock many insights about the material’s properties and behavior in reactions.
Calculating Limiting Reactants Using Moles
The concept of **limiting reactants and moles** is crucial in reaction calculations. When two reactants are combined, one may be used fully while the other is left over. By calculating the number of moles for each reactant, you can determine which one limits the reaction. For example, if you have 4 moles of A and 2 moles of B for the reaction A + 2B → C, A would be excess, and B would be the limiting reactant. Mastery of this concept can greatly improve the efficiency of chemical reactions in a lab setting.
Experimenting with Moles in the Laboratory
Practical experiments involving **mole calculations** can solidify your understanding of the concept. For instance, titration experiments often rely on accurately measuring moles to determine concentrations of unknown solutions. By knowing the volume and molarity of the titrant, you can accurately assess the amount of analyte in the solution by calculating it via mole relationships. Such hands-on experience with calculating moles leads to better retention of concepts and enhanced understanding of theoretical knowledge.
Key Takeaways
- Understanding moles is critical for performing accurate calculations in chemistry.
- Mole calculations can provide insight into chemical reactions and concentrations.
- Advanced mole concepts are vital for tasks such as determining limiting reactants and conducting accurate experiments.
FAQ
1. How can I convert moles to grams?
To convert **moles to grams**, utilize the formula: mass (g) = moles x molar mass (g/mol). For instance, if you have 2 moles of water (with a molar mass of 18.02 g/mol), your calculation would be: mass = 2 moles x 18.02 g/mol = 36.04 grams.
2. What is the significance of Avogadro’s number?
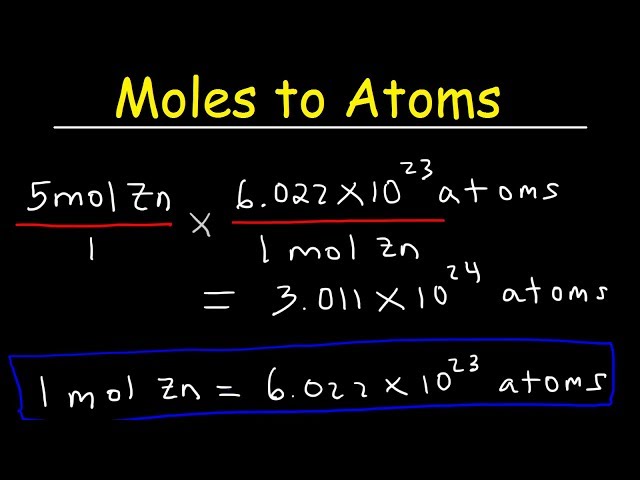
**Avogadro’s number** is crucial because it allows chemists to relate moles to physical quantities such as mass and volume. It signifies that one mole of any substance consists of \(6.022 \times 10^{23}\) individual entities, which is fundamental in particle counting and helps facilitate calculations in various chemical contexts.
3. How is molarity calculated for a solution?
**Molarity**, defined as moles of solute per liter of solution, is calculated as molarity (M) = moles of solute / volume of solution (L). This simple relationship is essential for understanding concentrations in various scientific applications.
4. What is the mole concept in stoichiometry?
The **mole concept** in stoichiometry enables the quantitatively balanced interaction between substances in a chemical reaction. By using mole ratios from balanced chemical equations, chemists can predict the amounts of reactants and products involved, transforming qualitative descriptions into quantitative predictions.
5. Can you explain the importance of moles in research studies?
Moles are integral in research as they allow for accurate measurements in a variety of experimental setups. This applies to fields such as biochemistry, materials science, and environmental studies, where determining precise concentrations is essential for drawing dependable conclusions from experimental data.
6. How can I calculate moles in real-life applications like cooking?
In cooking, understanding moles helps in relating the quantity of ingredients accurately. For example, knowing the moles of a particular ingredient can aid in scaling recipes effectively, particularly when dealing with chemical reactions in baking processes where proportionality is essential.