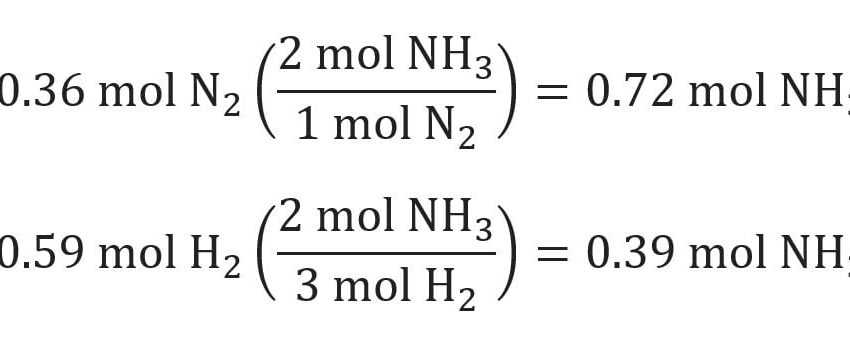Effective Ways to Find the Limiting Reactant in Chemical Reactions
Understanding how to find the limiting reactant in chemical reactions is essential for successful experimentation and proper stoichiometry. This guide will walk you through the definition of the limiting reactant, methods to identify it, and practical examples demonstrating its importance in reaction yield.
Understanding the Limiting Reactant
The limiting reactant is the substance that is entirely consumed in a chemical reaction, limiting the amount of product formed. This concept is crucial in chemical reactions because it helps predict the quantity of product that can form and determines the theoretical yield of the reaction. In any reaction using multiple reactants, one will be used up before the others, hence preventing further formation of the products and defining the reaction’s extent.
The Role of Balanced Equations
To accurately determine the limiting reactant, a balanced equation is essential. Balancing the equation ensures that you know the mole ratios necessary for the reaction to occur. Using the balanced equation, you can establish the relationship between the reactants. For instance, if a reaction requires 2 moles of Substance A for every 1 mole of Substance B, you will be able to identify how much of each substance is involved in the reaction and how it influences the product formation.
Stoichiometric Calculations and Mole Ratios
One effective method to find the limiting reactant is through stoichiometric calculations. By converting the amounts of your reactants from grams to moles using their respective molar masses, you can apply the mole ratio derived from the balanced equation. For example, if balances show that 3 moles of A react with 1 mole of B, and you have 6 moles of A and 1 mole of B, A is in excess, and B is the limiting reactant because it will run out first.
Practical Steps to Determine the Limiting Reactant
Finding the limiting reactant requires clear steps for accurate results. Here’s a structured approach to perform reactant calculations effectively:
1. Write and Balance the Chemical Equation
The first step to identifying the limiting reactant is to write the correct chemical equation. Ensure that it’s balanced, meaning the number of atoms for each element is equal on both sides of the equation. This is vital for performing any stoichiometric calculations accurately. Without a balanced equation, it becomes impossible to ascertain the mole ratios needed between the reactants and products.
2. Convert Reactant Amounts to Moles
Once the reaction is balanced, the next step is to convert the masses of the reactants into moles. This can be accomplished by using the formula:
- Moles = Mass (g) / Molar Mass (g/mol)
Make sure you measure the amounts correctly and use reliable values for the molar mass of each reactant. With the moles calculated, you can easily identify which reactant will be exhausted first during the reaction.
3. Determine the Limiting Reactant
Compare the actual mole amounts of each reactant to the coefficients from the balanced equation. By using these coefficients to assess how much product could be formed from each reactant, you can determine which one limits the reaction. For instance, if 2 moles of A can react with 1 mole of B, and you only have 0.5 moles of B available, then B is the limiting reactant.
Examples of Limiting Reactant Calculations
Let’s explore some limiting reactant examples to solidify your understanding.
Example 1: Simple Reaction
Consider the reaction:
C2H6 + O2 → CO2 + H2O
Imagine having 4 moles of C2H6 and 9 moles of O2. Balancing the equation yields:
2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
Using the coefficients, we find that 2 moles of C2H6 would require 7 moles of O2. Here, calculating for what’s available:
- For 4 moles of C2H6, we need 14 moles of O2.
Since only 9 moles of O2 are present, O2 is the limiting reactant.
Example 2: Complex Reaction
In a different reaction, you may react NH3 + O2 → N2 + H2O. Let’s say you have:
- 8 moles of NH3
- 4 moles of O2
The balanced equation shows you need 4 moles of O2 for every 2 moles of NH3. Analyzing the quantities gives us:
- 2 moles of NH3 need 4 moles of O2.
Thus, with 8 moles of NH3, you need 16 moles of O2. Since only 4 moles of O2 are available, it’s clear that O2 limits the reaction.
Key Takeaways
- Understand the definition of limiting reactant and its impact on reaction yield.
- Accurately balance chemical equations to determine mole ratios.
- Follow a systematic method for reactant calculations including conversion to moles and comparison with stoichiometric coefficients.
- Practice with various examples to solidify your understanding of how to apply these principles in real scenarios.
FAQ
1. What is a limiting reactant in chemical reactions?
A limiting reactant is the reactant that determines the amount of product that can be formed in a chemical reaction, as it is consumed first. Understanding which reactant limits the reaction helps in calculating the theoretical yield and ensures proper use of resources.
2. How can I easily find the limiting reactant?
To find the limiting reactant, first balance the chemical equation, convert all reactant amounts to moles, then use the mole ratios from the balanced equation to see which reactant will produce the least amount of product. This reactant is considered the limiting factor.
3. Why is it important to identify the limiting reactant?
Identifying the limiting reactant is crucial for accurate reaction calculations as it helps predict the maximum amount of product that can be formed, which is essential for both academic and industrial chemical reactions.
4. Can the limiting reactant change?
Yes, in different scenarios or with varying concentrations of reactants, the limiting reactant can change. If you use more of one reactant, you can alter which reactant limits the reaction efficiency.
5. What is the relationship between limiting reactants and theoretical yield?
The theoretical yield of a reaction is directly tied to the limiting reactant. It represents the maximum amount of product expected based on the amount of limiting reactant used. If you determine the limiting reactant correctly, you can effectively calculate the theoretical yield of your reaction.